We use cookies to offer you the best experience on our site. You can find out more about the cookies we use or disable them in the settings. Cookie settings
RAPHAELA ACG
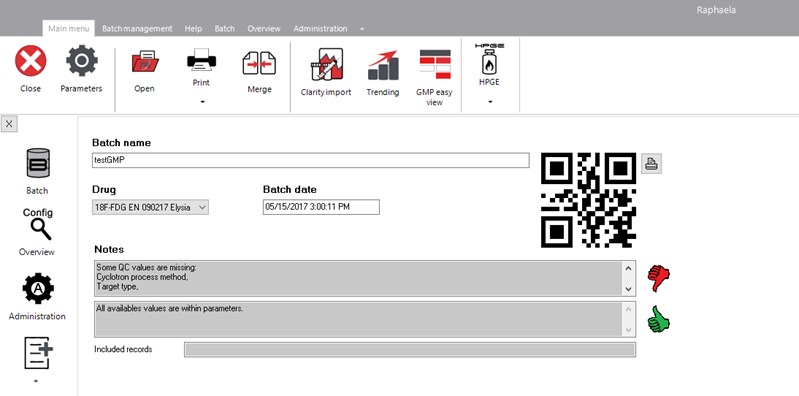
The Elysia-raytest RAPHAELA ACG (previous name was BARES Batch recording & reporting system) is a data base LIMS system to acquire, record & report results from the individual instruments applied for radiopharmaceutical production and QC. It records automatically and digitally the QC information from the different measurement instruments required by law for the release of a radio-pharmaceutical tracer.
The software is preconfigured for 18F-FDG, but can also be easily used for other PET tracers like 18F-FEC, 18F-FET, etc. It can also collect data from the cyclotron, synthesis module, QC instruments & dispensing unit. It assembles automatically all information to one common analysis report. This contains the measured value and information regarding the level of acceptance. It also remarks if one value is out of the acceptance criteria or the required value is missing. The final report will be digitally signed when released by the responsible person.
The RAPHAELA ACG runs on a separated server computer system. It uses a database to store all QC instrument information, QC result values & dispensing information (if available). The RAPHAELA ACG system can be configured individually according to the local authorities’ requirements and regulations. Each required value of the individual analytical QC instrument will be transferred digitally and automatically controlled, decreasing drastically risk of data miscopying due to human operator.
The configuration of RAPHAELA ACG contains the level of release acceptance. If the received value is out of that level, the release will be refused. The required entries can be configured. For very specific customer requirements, manual entries can be added (marked as manual entry). The user management describes the different levels of users & their authorization up to the authorization to sign (release) the product. Also the configuration can be country & language specific.